G
protein coupled receptors (GPCRs) are the most important drug targets
for modern drug discovery. GPCRs are involved in different cell
signalling including inflammation, neuron transmission, cell growth
and many others. Almost all human diseases and disorders are related
to GPCRs and their signalling. GPCRs have become popular targets for
modern drug discovery and about 40% of the marketed drugs are
targeting them.
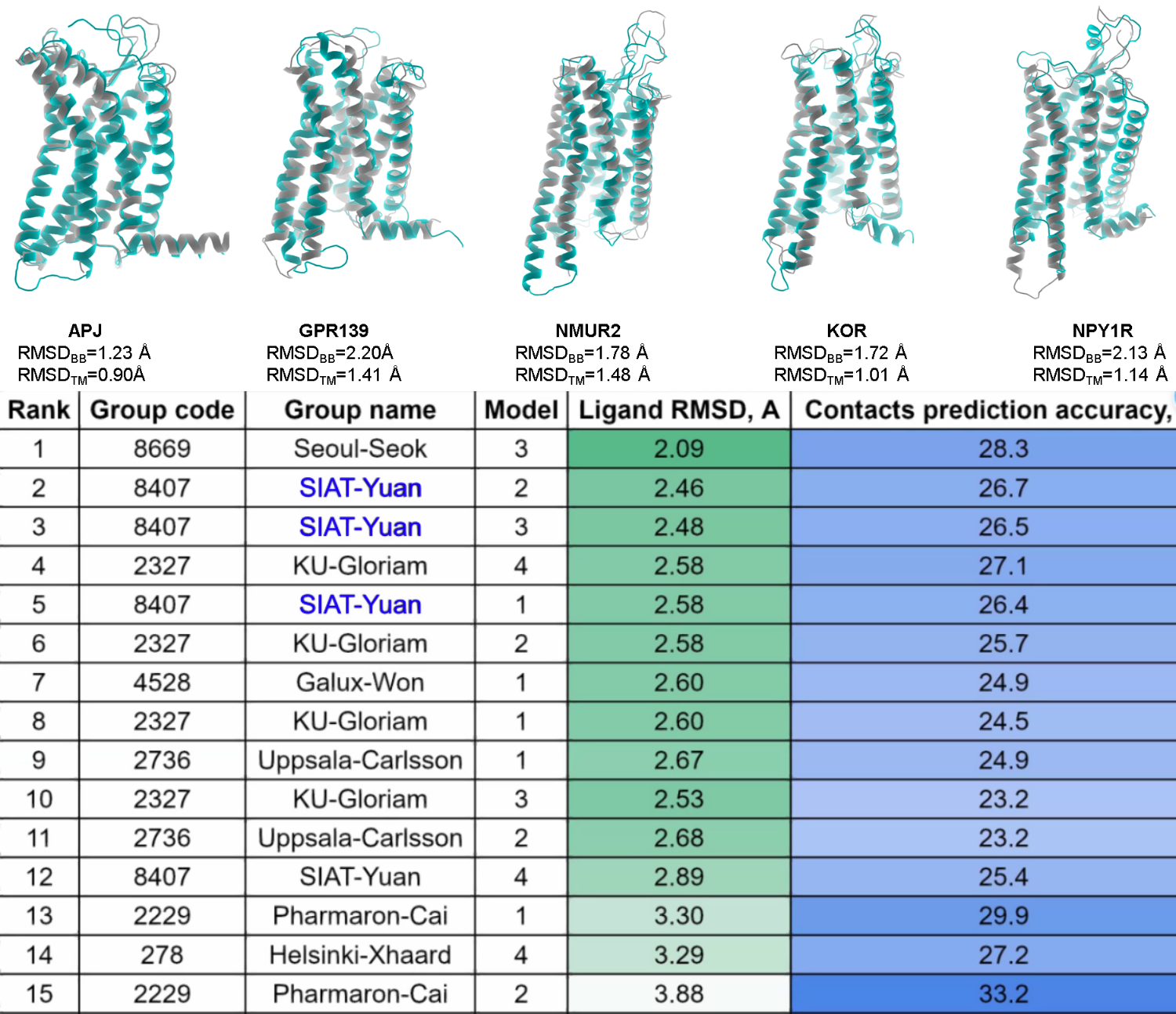
The recent international GPCR-DOCK 2021
took place in December, 2021. The purpose of this event is to
validate the accuracy of modern computational methods of the model
predictions for GPCRs and their ligand binding. There were 45 groups
from all over the world joint this contest. Five new GPCR targets,
including GPR139, NPY1R, APJ, NMUR2 and kOR receptors, as well as
their ligand were provided to predict their 3D models as well as
their binding modes. More than 800 models in all were submitted for
this contest. The results of this global event were announced during
April 13-14 in the 7th iHuman Forum symposium. The cofounder of
AlphaMol Dr. Yuan was invited to give a presentation in this event to
share our successful story.

Prof.
Irina Kufareva from UCSD, who is an organizer of GPCR-DOCK 2021,
concluded that the difficulty of modelling for GPCR-DOCK 2021 is:
GPR139
Based
on the newly resolved GPCR structures, comparisons were also made
between the predictions of AlphaMol and AlphaFold2, as well as
that with RosettaGPCR. comparison results indicated that the
predictions from our team were much more accurate than
both AlphaFold2 and RosettaGPCR ,
of which both models were obtained from their official deposit
website.
A
news feature article, which
is entitled "What's next for AlphaFold and the AI
protein-folding revolution" has been published on April 13, 2022
in Nature. In this paper, Prof. Brian Roth, who is a structural
biologist and pharmacologist at the University of North Carolina at
Chapel Hill, stated the following:
...
"AlphaFold isn’t always that accurate. Of the several dozen
GPCR structures his lab has solved, but not yet published, he says,
“about half the time, the AlphaFold structures are fairly good, and
half the time they’re more or less useless for our purposes”. In
some instances, he says, AlphaFold labels predictions with high
confidence, but experimental structures show that it is wrong. Even
when the software gets it right, it cannot model how a protein would
look when bound to a drug or other small molecule (ligand), which can
substantially alter the structure. Such caveats make Roth wonder how
useful AlphaFold will be for drug discovery." ...
One
major reason for why AlphaFold2 doesn't perform perfectly for GPCR
could be: there are about 190 K structures of macromolecule were
deposited in the PDB database, of which the proportion of membrane
protein is only about 4%. Most of the current resolved structures
were soluble proteins which occupied about 91%. The rests were
nucleotide. AlphaFold2 is an AI-based tool which highly depends
on the size of training data. However, the number of resolved
membrane protein structures are only about 6500 in all. Our new
algorithm is not pure AI-based and overcome this weakness.
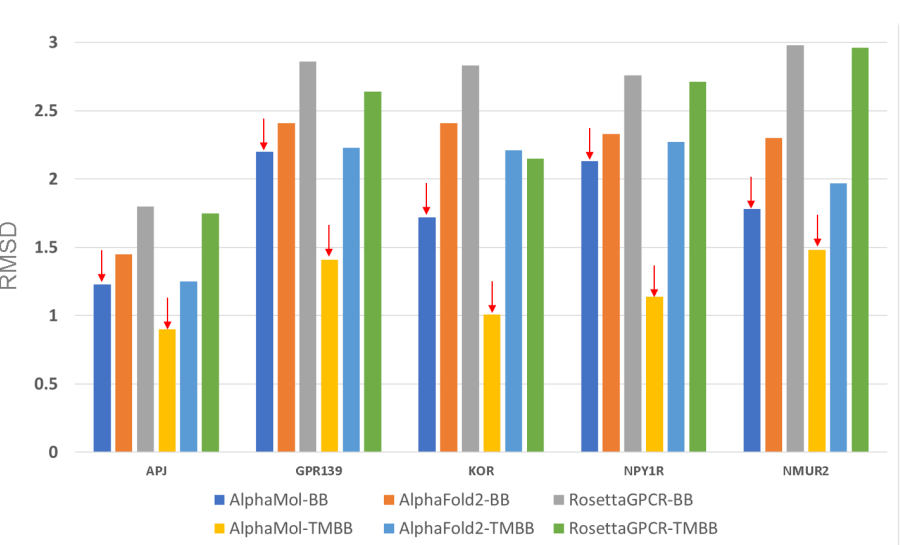
Prediction
comparisons were made among AlphaMol, AlphaFold2 and RosettaGPCRs for
the new five GPCR targets from GPCR-DOCK 2021. The models from
AlphaMol have much smaller RMSD of GPCR backbones and transmembrane
(TM) helices with experimental structures than that from both
AlphaFold2 and RosettaGPCRs. Smaller RMSD indicates higher
accuracy.