On September 3, 2024, the team of AlphaMol Science Ltd, in collaboration with the Max Planck Institute in Germany, CADD center at the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, and the School of Pharmaceutical Sciences at Shenzhen University of Science and Technology, published a research article titled "Structure of Tetrameric Forms of the Serotonin-gated 5-HT3A Receptor Ion Channel" as the cover story in the top international academic journal - The EMBO Journal.

The serotonin ion channel 5-HT3A receptor (5-HT3AR) is an important drug target, closely related to diseases such as depression, vomiting in cancer patients undergoing radiotherapy and chemotherapy, and pains. The cryo-electron microscopy structure of the 5-HT3AR in a native cellular membrane physiological environment was resolved in this work. Surprisingly, in addition to the traditional pentameric structure, the target was also found to form a tetrameric structure. What's more, this tetrameric structure presents in two different states as "symmetric tetramer" and "asymmetric tetramer".

Figure 1. Snapshot of the article online.
The team elucidated the molecular mechanism of the receptor's assembly from monomers to multimers in a physiological environment through computer simulation systems. Computer simulations and biochemical experiments showed that the pore space formed by a single 5-HT3AR tetramer is too narrow to accommodate the passage of ions through the channel to perform its physiological roles of signal transduction. The computer simulations also found that the formation of a pentamer from a monomer combined with a tetramer is energetically more favorable than the formation of a pentamer from a dimer combined with a trimer.
This research paper provides new insights and theoretical guidance for understanding the physiological function of 5-HT3AR and designing 5-HT3AR antagonistic drugs.
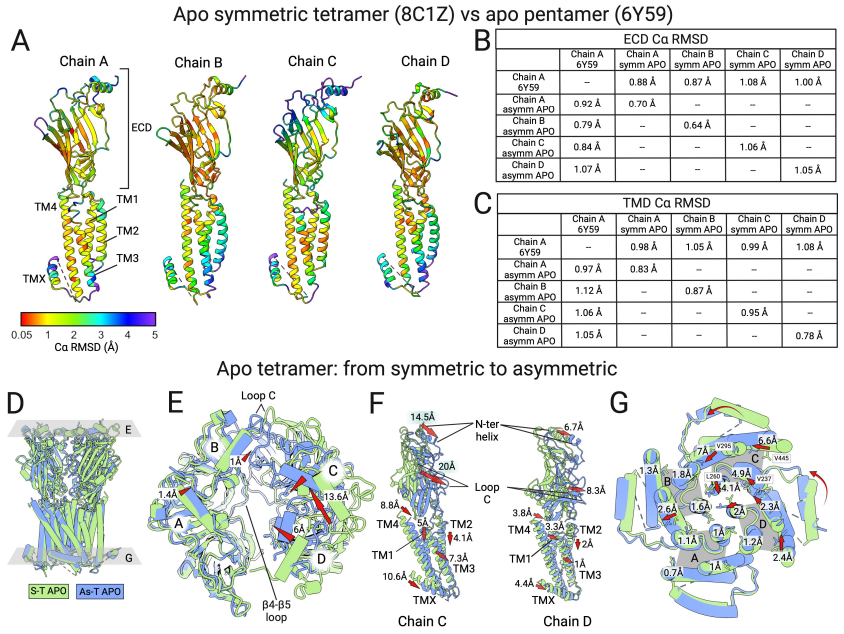
Figure 2. Structural comparison of the tetrameric and pentameric forms of the 5-HT3A receptor.
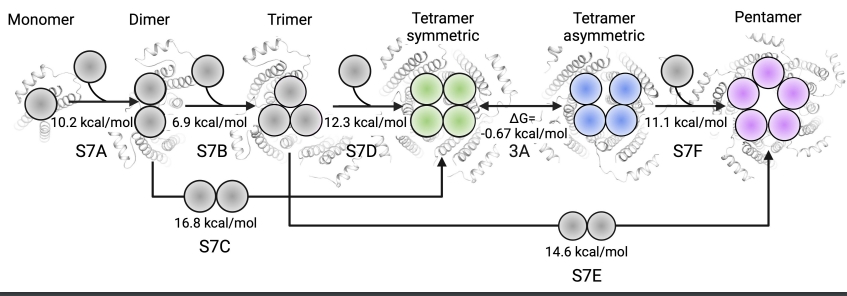
Figure 3. Model of the pentameric assembly pathway.
The corresponding authors of this paper are Professor Mikhail Kudryashev from the Max Planck Institute, Professor Yuan Shuguang and Professor Horst Vogel from AlphaMol Science Ltd. The co-first authors are Dr. Bianca Introini from the Max Planck Institute, Dr. Cui Wenqiang, a 2023 graduate of the University of Chinese Academy of Sciences, and Dr. Xiaofeng Chu from the Helmholtz Center for Molecular Medicine.
Link to the full text of the paper:
https://www.embopress.org/doi/full/10.1038/s44318-024-00191-5